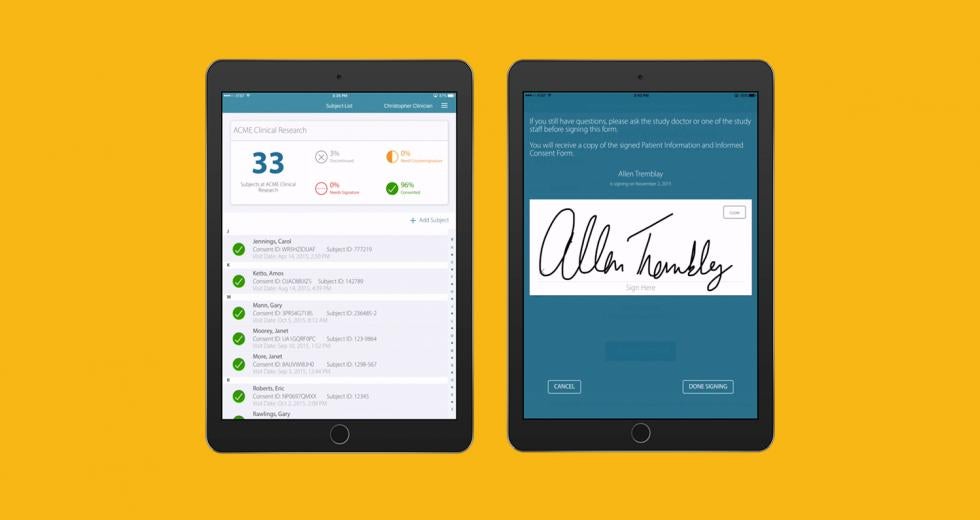
Anthony Costello had the next generation of medicine in mind when he launched Mytrus in 2009. The Davis-based company created a system that helps patients fill out consent forms electronically and participate in clinical trials for new drugs and therapies from the comfort of their homes.
The technology has huge potential for both patients and the pharmaceutical industry, not only in America but worldwide. By eliminating paperwork, the system cuts costs and saves time, helping carry health IT into the 21st century. Six years after stepping into medtech’s competitive arena, Mytrus boasts eight of the top 10 big pharma companies as customers. Next month, the company will begin working with the Patient Centered Outcomes Research Institute and Duke University on a clinical trial touted as the largest virtual remote study ever.
How did Mytrus succeed where so many others have failed?
SLOW TO EVOLVE
You’ve read the claims: Brain implants could restore memories for Alzheimer’s patients. Smart armbands monitor hearts. Computer to print 3D human organs. Every other day, a high-tech startup comes forward with a futuristic device or interactive app designed to transform how we live and thrive in the digital age. With all these advances, you’d think there would be an easier way to collect data from clinical trials. But most hospitals remain stuck in the past, using antique tools (i.e. pens and paper) to monitor performance.
“It’s so labor intensive to gather and score these forms,” says Dr. Eric Klineberg, who is an adult and pediatric spinal surgeon at the UC Davis Medical Center. “If a patient, instead of circling a box, selects ‘this question does not apply’ or doesn’t fill in a circle completely, the data cannot easily be scored or used to generate an outcome measure. It seems like it’s crazy in this day and age, that we are still filling out any of these types of forms on paper. I don’t even think I do it at the DMV anymore.”
The medtech arena is complex. Unlike other industries, health IT companies are caught between the drive for innovation and the demands of privacy. Successful ones figure out how to make a state-of-the-art solution without sacrificing cybersecurity. But this is a nuanced challenge, which is why a 2013 report by PricewaterhouseCooper’s Health Research Institute found medtech executives remain behind the adoption curve — slower to apply social, mobile, analytic and cloud technologies.
“Half of medtech companies appear to be using such technologies to at least some degree to engage customers and patients in managing their health and to enable remote monitoring,” the report says. “But only 12 percent are using them aggressively to create new business models that center on clinical and consumer dynamics. With outdated and unresponsive information technologies, companies might miss opportunities to meet the needs and demands of the next generation of consumers.”
MAKING MOVES
Mytrus started in San Francisco, but in 2013, Costello relocated to Davis. He grew up in Davis and says the area is an untapped medtech goldmine due to the presence of major health systems: Kaiser, Dignity, Sutter Health and the UC Davis Medical Center. Still, the move could be considered counter-intuitive since the Capital Region lacks the same funding opportunities as the Bay Area.
“We have a great ecosystem for health care and medical devices, but there is currently a lack of funding here for some of the innovation companies to stay,” says Matthew Phillips, director of the MedStart program for the Sacramento Regional Technology Alliance. “There are a few venture capital groups that have helped companies successfully. The majority of the money is based in the Bay Area. It’s more expensive to live there and start a company, but that’s currently where most of the money is.”
But Costello knew his company had a solid foundation. Its co-founders have career experience in medtech or medicine from the likes of Berkeley, Stanford, the UC San Francisco and various clinical research companies. The current team of 20 consists of medical professionals and engineers who live in Davis, Sacramento, the Bay Area or the company’s Texas office in Austin.
Up to this point, Costello says Mytrus’ strategy has been a basic one: Listen to the customers and shift accordingly. Mytrus initially began with a primary focus on remote clinical data collection. The company even bought a patent from Boston University for exclusive rights to run virtual trials in the U.S. But it was clear after a year that most pharmaceutical companies were simply not yet ready for remote participation. Health care providers first wanted a simple way to avoid paper forms and allow patients to give consent electronically. Mytrus obliged.
If you can survive long enough and pivot at the right times, sometimes you can hone in on what customers are really needing — whether or not they even realize it yet. You can’t ignore signals about what your customers really want to buy.” Anthony Costello, CEO, Mytrus
“That was a big pivot, but it helped us grow our market share,” Costello says. “Immediately, we became a leader in this new technology and started getting more serious looks from big pharmaceutical companies. If you can survive long enough and pivot at the right times, sometimes you can hone in on what customers are really needing — whether or not they even realize it yet. You can’t ignore signals about what your customers really want to buy.”
Many companies go through product development phases before actually releasing a product. Mytrus spent 18 months in product development before they launched their first FDA-approved study with Pfizer. Since then, significant trends toward technology in clinical trials and help from two rounds of investment have allowed Mytrus room to expand R&D on several “first-mover” technologies.
REMOTE CONTROL
Typically, patients involved in clinical trials for new drugs go to hospitals for check-ups every few months. They fill out forms to answer physicians’ questions about their health status: How are you feeling? Are you happy? Any physical reactions? Changes in sleeping patterns? Beyond the hospital, communication is tricky. Because the data is sensitive and private, extremely secure systems must be used, but these systems are not easy or cheap to develop.
“It’s a difficult industry to work in from an innovation standpoint,” Costello says. “We badly need innovation because drug research is so slow and expensive. Lots of technology has been introduced into this industry over the last 10 years, but very little of it is well-suited for patients to be the users outside a hospital setting.”
With Mytrus, Costello sought to put the technology in patients’ hands, allowing them to provide data remotely and better understand what was going on with research. No longer would they need to come into the hospital just for periodic data collection, since physicians can monitor them and check their data remotely through Mytrus’ web-based technology.
It’s not hard to see the appeal of such a system. Another key finding of PwC’s medtech study was that the value of a device is no longer just about the product. While clinical efficiency is critical, the report says the most innovative companies offer solutions that “solve additional problems such as improving diagnostics, increasing operating room efficiency, reducing length of hospital stays, monitoring patients remotely and keeping people out of the hospital.”
Next month, Duke will launch a clinical trial enrolling 20,000 patients. All of them will sign consent electronically with Mytrus’ system. They will then be randomized by Mytrus into one of two doses of Aspirin and followed for long-term cardiac outcomes. Every month for two years, patients will get a text or email prompting them to go online and fill out the study forms in the Mytrus-secured system. This huge project, Costello says, will demonstrate how to drastically reduce costs through remotely collecting patient data outside of a hospital setting.
This is the kind of technology that can transform the industry. But for physicians, the one true measure of success is the level of security, which must be the first priority.
“People are breaking in and are able to steal social security numbers from Target,” says Klineberg, who is waiting for regulatory approval to implement online forms. “Any health information must be protected, and the worst thing I can do is violate patient confidentiality. We need to ensure our online security system is safer than others, and we need to only collect outcome information to protect our patients.”
INNOVATION WITHOUT BORDERS
Privacy is the number one concern when it comes to patient data. This is true in the U.S. but an even bigger deal around the globe, where regulations can vary widely. Countries who have approved or will soon approve the Mytrus system include Brazil, Hungary, England, India and Canada. Each country has data privacy nuances and sometimes complications arise.
In a recent study in India, many of the patients were illiterate. Mytrus built an iPad app to allow patients to listen to the content using headphones, and sign with fingers and a thumbprint if they could not write. This is groundbreaking in many ways, Costello says, because now patients in developing countries can receive medical treatments they might not have access to otherwise.
Phillips says any IT-based technology that can reduce costs to health providers and increase access to health care for patients will be the game-changers moving forward. This includes cloud-based resources, electronic signatures and virtual trials.
“It’s growing because there’s a need,” he says. “Everybody’s clamoring for a higher degree of technology to help lower costs. You have to have a product that can provide better health care, save a life and save money — a disruptive technology that can get people better access and not just, ‘Hey, I’ve got this cool gadget that can do something neat, what do you think of it?’”